Boron isotopes in Marine Carbonates
The Ph Proxy - The Basics
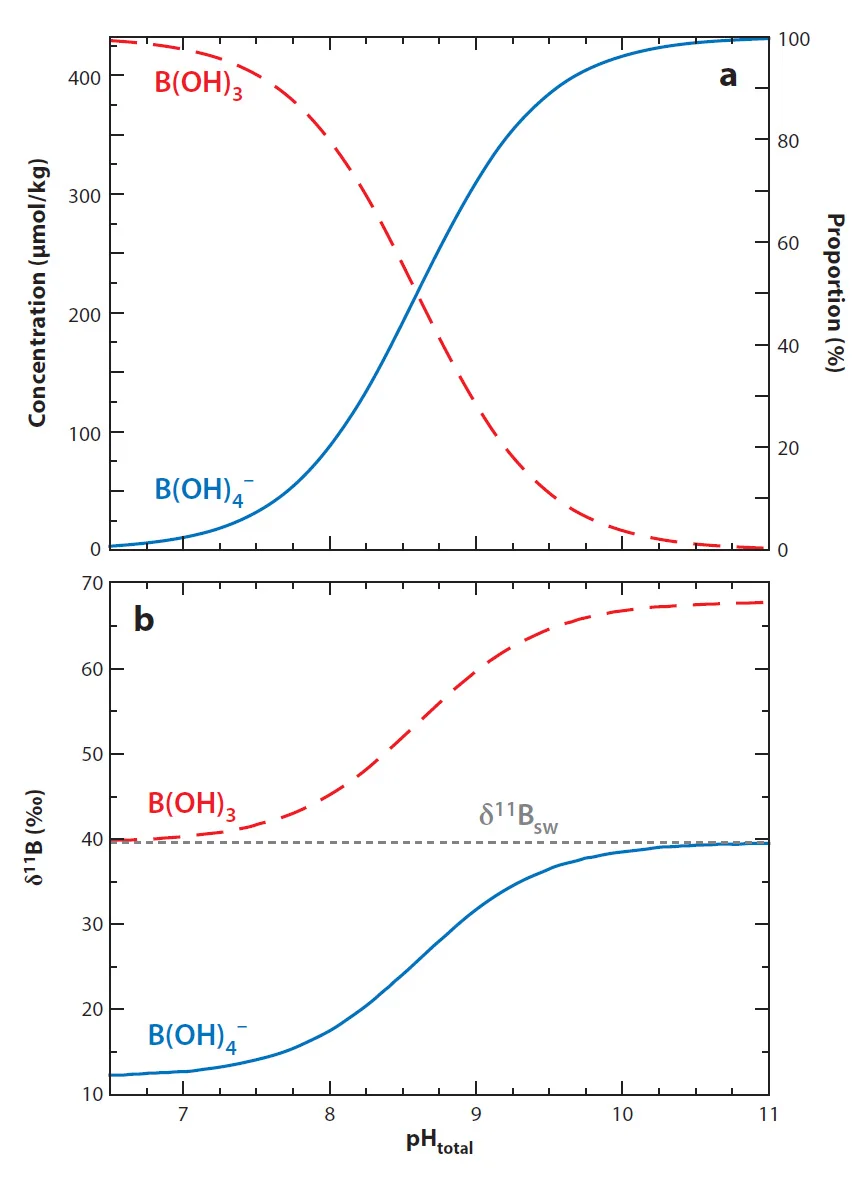
Seawater contains around 4.5 ppm boron (B) and it exists in two forms as function of pH: boric acid (B(OH)3) and borate ion (B(OH)4-; top). Boron has two isotopes: 11B and 10B (80:20 ratio) and due to structual differnces there is a pronouced isotopic fractionation between the two aqueous forms. Boric acid has a higher 11B/10B ratio than borate ion by 1.0272 (Klochko et al., 2006). As pH changes the abundance of each aqueous species changes and so too does their isotopic composition (bottom). The proxy is based on the observation that predominantly only borate ion is incorporated into marine calcium carbonate (Hemming and Hanson, 1992).
Ph to CO2
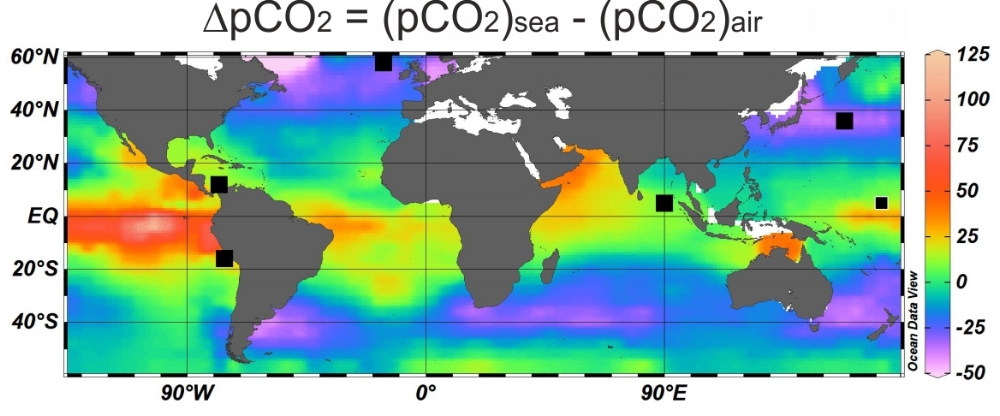
The boron isotopic composition of ancient foraminifera that lived in the surface mixed layer offer a means to reconstruct ancient seawater pH. Seawater pH, like CO2, is determined by the ratio of total dissolved carbon (DIC) to total alkalinity (ALK; top). Given the nature of the CO2-system in seawater an additional parameter of the system is however needed for quantitative estimates of seawater CO2 content. Once seawater CO2 content is reconstructed air-sea disequilibrium with respect to CO2 then needs to be accounted for (bottom). Careful selection of study sites can minimise the influence of this requirement on the final CO2 reconstructed.
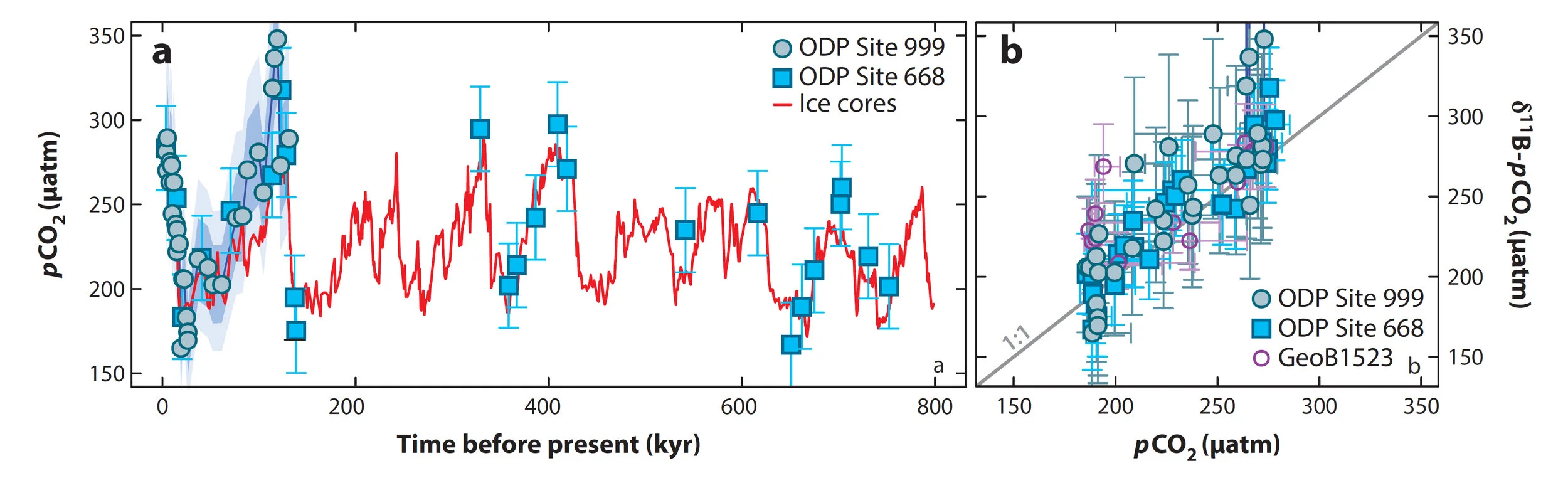
Existing attempts at validation of the boron isotope proxy from Foster and Rae (2016) including data from Hoenisch et al. (2009)
Potential complications
There are a number of other critical assumptions to calculate CO2 from boron isotopes:
- The boron isotopic composision of seawater needs to be known (e.g. Lemarchand et al. 2002).
- Minor "vital effects" relating to foraminiferal physiology need to be corrected for (e.g. Henehan et al. 2016).
- The forams should be well preserved (Seki et al., 2010; Honisch & Hemming, 2004), though diagenesis is not a significant problem (Edgar et al,. 2015).
Existing Validation Efforts
There have been a number of attempts to validate the boron isotope proxy against the ice-core CO2 record (summarised in Foster and Rae, 2016, see figure above). There is clearly a good agreement with the average residual for the data shown above being 23 μatm: clearly demonstrating that for the Late Quaternary the boron isotope proxy is able to accurately reconstruct atmospheric CO2 variations. These results support the use of boron isotopes to provide accurate estimates of pCO2 beyond the reach of the ice cores (the last 800 kyr).
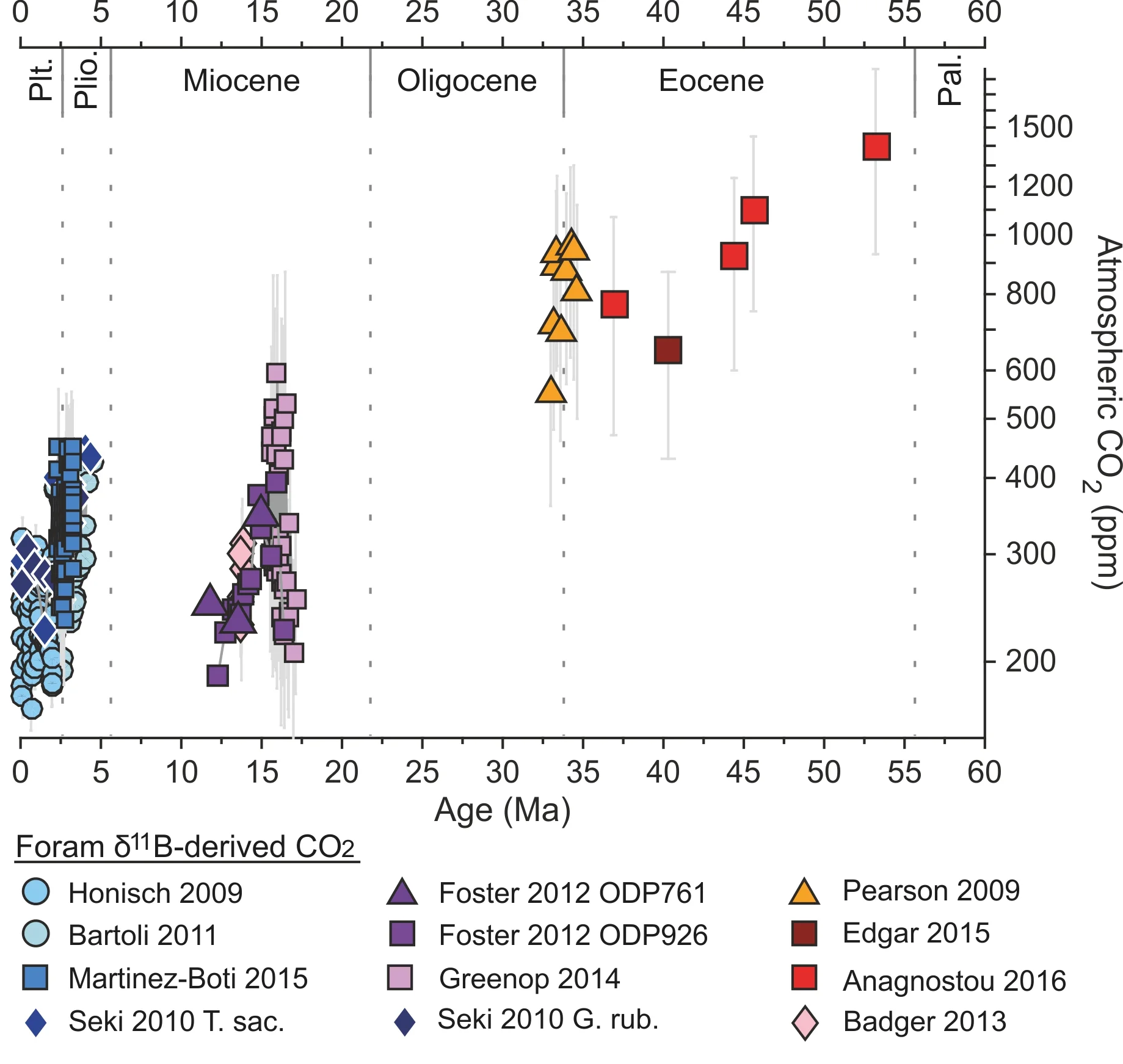
Cenozoic CO2 using Boron isotopes
Summary of boron isotope derived CO2 for the last 65 million years. Click here (insert link) for data in the diagram above. The data clearly show that CO2 has, on the whole, decreased over this interval, with a peak of ~1400 ppm during the Early Eocene.
Rejected datasets
The following datasets have been rejected from thecompilation due to advances in the understanding of the isotopic fractionation factor, improved estimates of the second carbonate system parameter, and advances in analytical techniques:
Spivak et al. (1993); Sanyal et al. (1995); Pearson and Palmer (2000)
B/Ca and why we don't Use it
Because the concentration of borate ion is pH dependent and it is predominantly borate that is included into foraminifera, the concentration of boron (expressed as B/Ca ratio) should also be pH dependent. In benthic forams it works well as a a carbonate ion proxy (see Yu and Elderfield, 2007), but in planktics there seems to be a myriad of other controls (see Allen et al., 2012; Henehan et al., 2015; Uchikawa et al., 2015). It is therefore too early to use B/Ca as a quantitative proxy of CO2.